[Last Update: June 28th, 2018]

|
D. Jon Scott’s Website ► Science ► Physics ► Chemistry ► Organic Chemistry ► Prebiotic Synthesis & AbiogenesisPrebiotic Synthesis Experiment:
Copyright © 2018 by Dustin Jon Scott
|
| Compound Class | Subclass | Compound | Source |
|---|---|---|---|
| Amino Acids 17-60 ppm | Alanine | Murchison meteorite | |
| Glutamic acid | Murchison meteorite | ||
| Glycine | Murchison meteorite | ||
| Pseudoleucine | Murchison meteorite | ||
| Isovaline | Murchison meteorite | ||
| Hydrocarbons | Aliphatic >35 ppm | Murchison meteorite | |
| Aromatic 3319 ppm | Murchison meteorite | ||
| Fullerenes >100 ppm | Murchison meteorite | ||
| Carboxylic acids >300 ppm | Murchison meteorite | ||
| Hydrocarboxylic acids 15 ppm | Murchison meteorite | ||
| Alcohols 11 ppm | Murchison meteorite | ||
| Nucleobases 1.3 ppm | Purines | Adenine | Murchison meteorite |
| Guanine | Murchison meteorite | ||
| Xanthine | Murchison meteorite | ||
| Pyrimidines | Uracil | Murchison meteorite | |
| Alkyl phosphonic acids 2 ppm | Ethylphosphonic acids | Murchison meteorite (Cooper &al., 1992) | |
| Methylphosphonic acids | Murchison meteorite (Cooper &al., 1992) | ||
| Alkyl sulfonic acids 68 ppm | Murchison meteorite (Cooper &al., 1992) | ||
| Silicates | CM group & CI group carbonacous chondrites. | ||
| Oxides | Dihydrogen monoxide 3-22% | CM group & CI group carbonacous chondrites. | |
| Sulfides | CM group & CI group carbonacous chondrites. | ||
| Phosphates | Apatite | (Schwartz, 2006) | |
| Inorganic phosphate | Detected in the Murchison meteorite at about 25 micromoles per gram (Cooper &al., 1992) | ||
| Inorganic orthophosphate | Murchison meteorite (Cooper &al., 1992) | ||
| Schreibersite [(Fe, Ni)3P] | (Schwartz, 2006) | ||
| Whitlockite [Ca9(Mg, Fe)(PO4)6PO3OH] | (Schwartz, 2006) | ||
| Chlorapatite [Ca5(PO4)3Cl] | (Schwartz, 2006) | ||
| Aluminous spinel | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Aluminum | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Anorthite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Calcic pyroxene | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Calcium | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Fosterite-rich olivine | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Hibonite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Melilite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Perovskite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. |
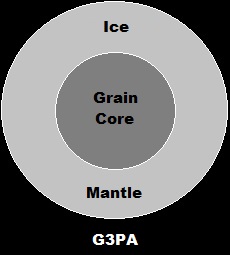
Part II.a-2.
Ice Mantle Composition
| Compound | Source |
|---|---|
| Cytosine | Experiments with astrophysical ice analogues. |
| Dihydrogen monoxide | |
| Purine | |
| Pyrimidine | |
| Ribose | Experiments with astrophysical ice analogues. |
| Thymine | Experiments with astrophysical ice analogues. |
Part II.b.
Atmosphere
Should be a reducing hydrogen-rich atmosphere.
Part III
Methods
The grains should vary considerably in the proportions of their composition. The grains should be placed in some kind of a transparent tray that can be irradiated with UV light from below.
Part IV
Projected Results
Hypothetically, the sublimation of H2O ices within the grain cores of the G3PAs will free other compounds that may react with one another in novel ways and perhaps form interesting bonds as these compounds re-settle during recondensation. Repeating this many times with many different G3PAs, we may be able to emulate the accretion of protoplanetary grains into larger bodies, as the freeing and re-settling of compounds transforms discrete grains into homogeneous strata.