[Last Update: June 28th, 2018]

|
D. Jon Scott’s Website ► Science ► Physics ► Chemistry ► Organic Chemistry ► Prebiotic Synthesis & AbiogenesisPrebiotic Synthesis Experiment:
Copyright © 2018 by Dustin Jon Scott
|
1. |
The half-lives of the nucleobases guanine, adenine, cytosine, and uracil are far too short (t½ for adenine and guanine ≈ 1 year; uracil = 12 years; cytosine = 19 days) at the 80-110°C temperatures hypothesized in hydrothermal vent scenarios for the origin of life (based on the preferred temperature ranges of hyperthermophiles, assumed by hydrothermal vent scenarios to be among the oldest extant lineages of living things), and indeed are too unstable at temperatures much above 0°C, to allow for the formation of the first replicators in a reasonably long timespan, indicating a low-temperature origin of life (Levy & Miller, 1998). |
2. |
RNA hydrolyzes rapidly (Szostak, 2012; part 3), and is especially prone to doing so in hot environments, much to the chagrin of those who favor hydrothermal vent models for the origin of life. |
3. |
While hydrothermal vent hypotheses imply a hyperthermophilic progenote with an optimal growth temperature (OGT) ≥80°C, which is consistent with thermophilia (OGT=65±15°C) in the last bacterial ancestor as well as the last archaeal ancestor as part of a more-or-less linear progression to the overwhelmingly mesophilic (OGT≤50°C) modern prokaryotic domains, dual phylogenic rRNA and protein analyses show that while both the bacterial ancestor and the archaeal ancestor were thermophilic (OGT=65°C±15°C), the LUCA was mesophilic with an OGT≤50°C (Boussau et al., 2008). |
4. |
The very first protocells were likely obligate cryophiles with an OGT≈1°C (Szostak, 2012; part 1) |
The hydrothermal vent model, though seemingly ruled out, would give us a mathematically elegant and thermodynamically sensible sequence of OGT≥80°C, OGT=65±15°C, OGT≤50°C and a cellular LUCA model gives us a progressive OGT≈0°C, OGT≤50°C, OGT=65±15°C thermotolerance sequence before a general decline to OGT≤50°C with a few modern lineages OGT=65±15°C or even OGT≥80°C, while a precellular LUCA model merely gives us an OGT≤50°C; OGT≥50°C threshold separating the cryophilic-to-mesophilic nucleobases, RNA, LUCA, and protocells, and the thermophilic-to-hyperthermophilic first true lifeforms, followed eventually by diversification into various levels of thermotolerance.
Cool Early Earth model.
Here, however, we run into another problem. Water is highly corrosive, and would've been deadly to the first RNA-based replicators (Barras, 2014)
Hydrolysis (Benner et al., 2012)
Part I.b.
Molecular Panspermia
The earliest stages of life on Earth very likely antedate the Earth Herself. This idea has been variously referred to as “molecular panspermia", “quasi-panspermia", or “pseudo-panspermia".
"Essential to the spontaneous origin of life was the availability of organic molecules as building blocks. The famous ‘prebiotic soup’ experiment by Stanley Miller (Miller 1953, Miller-Urey experiment) had shown that amino acids, the building blocks of proteins, arose among other small organic molecules spontaneously by reacting a mixture of methane, hydrogen, ammonia and water in a spark discharge apparatus. These conditions were assumed to simulate those on the primitive Earth. Already in 1922 Oparin had proposed that the early Earth had such a reducing atmosphere (in his classic ‘The Origin of Life’ from 1936 he expanded on these ideas). Observations of Jupiter and Saturn had shown that they contained ammonia and methane, and large amounts of hydrogen were inferred to be present there as well (it is now known that hydrogen is the main atmospheric component of these planets). These reducing atmospheres of the giant planets were regarded as captured remnants of the solar nebula and the atmosphere of the early Earth was assumed by analogy to have been similar." -- The Origin of Life by Albrecht Moritz
The conditions of the Miller-Urey experiments more closely resemble the conditions of the Solar nebular than the conditions of the primordial Earth (Hill & Nuth, 2003).
"Indigenous purines and pyrimidines have been detected in several carbonaceous chondrites. The pyrimidine uracil and the purines adenine, guanine, xanthine, and hypoxanthine (Stoks & Schwartz 1979, 1981) were detected in the CM carbonaceous chondrites Murchison and Murray, as well as in the CI meteorite Orgueil, in total concentrations of about 1.3 parts per million (ppm). Upper limits exist (detection limit of 0.01 ppm) for the concentrations of thymine and cytosine, as well as other heterocyclic compounds, in the Murchison meteorite (van der Velden & Schwartz 1977)."
(Peeters et al., 2003)
The purine bases adenine and guanine have been detected in meteorites, although the only pyrimidine-base compound formally reported in meteorites is uracil (Stoks & Schwartz, 1979), however cytosine cannot be ruled out (Shapiro, 1998; Peeters et al., 2003; Martins et al., 2006) and ultraviolet irradiation of low-temperature ices (the dominant phase of H2O in cold astrophysical environments is ice, and most ices in such environments are H2O-rich) has been shown to produce not only amino acids, quinones, and amphiphiles, but have also, with the introduction of pyrimidine, been shown to produce uracil (Nuevo et al., 2009), cytosine, and even thymine, though the abiotic synthesis of thymine is less straightforward compared to other pyrimidine-base compounds (Sandford et al., 2014). (That this would logically make the prebiotic synthesis of RNA easier than that of DNA could explain why RNA has a larger repertoire of functions than does DNA in modern cells.) Additionally, ribose and related sugars have been produced experimentally in astrophysical ice analogues (Meinert et al., 2016).
Cytosine can be synthesized from cyano-acetylene and cayanate, this is unlikely to have occured in acqueous media as cayanate is rapidly hydrolized into CO2 and NH3.
Cyano-acetylene is an abundant interstellar molecule and can by produced by a spark discharge in a CH4/N2 environment.
hydrolysis of cyanoacetylene leads to cyanoacetaldehyde
reaction of cyanoacetaldehyde with urea produces cytosine in 30-50% yields
Hydrolysis of cytosine leads to uracil
Organic methyl cyanide in comet-forming region of MWC 480.
Clear liquid turns brown as amino acids form peptides. Jennifer Blank. Experiment.
Amino acids + impact = peptides (Blank & al. 2001)
Planetesimals & chondrules
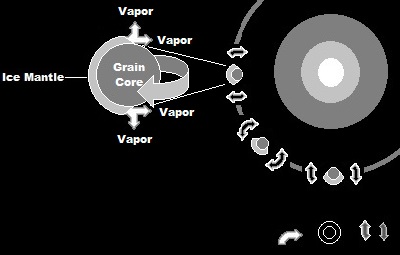
Being that ribonucleotides can form sugar-phosphate backbones in clays when frozen (cite) we should consider whether it be possible that the first RNA molecules were produced by the sublimation/condensation cycles of clayey, H2O-rich spinning grains along the snowline of the protoplanetary disc.
Figure out minimum grain size for clay sheet formation.
Ribonucleic peptide (PNA), uses peptides instead of phosphorylated ribose.
Part I.c.
The Protoplanetary Origin of Life
What all of this means is that hypothetical geochemical pathways for the production of key organic compounds here on Earth, though interesting, are completely superfluous, since the biochemical building blocks of life were already being produced astrochemically before the formation of the Earth. Occam's razor therefore dictates that such geochemical hypotheses for the prebiotic synthesis of organic compounds should be regarded as irrelevant to the origin of life problem.
Part II
Materials
This experiment should employ granular protoplanetary particle analogues (GPPPAs or G3PAs) mimicking the ice-mantled grains that would've existed on the snowline of the protoplanetary Solar disk, with each G3PA consisting of a clayey, silicate-heavy grain core surrounded by an H2O-rich ice mantle.
Part II.a.
Granular Protoplanetary Particle Analogues
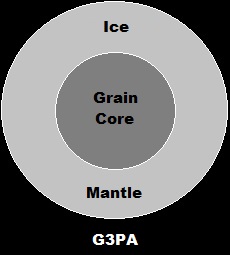
Part II.a-1.
Grain Core Composition
Grain composition — clays/silicates, adenine, guanine, pyrimidine, uracil.
| Compound Class | Subclass | Compound | Source |
|---|---|---|---|
| Amino Acids 17-60 ppm | Alanine | Murchison meteorite | |
| Glutamic acid | Murchison meteorite | ||
| Glycine | Murchison meteorite | ||
| Pseudoleucine | Murchison meteorite | ||
| Hydrocarbons | Aliphatic >35 ppm | Murchison meteorite | |
| Aromatic 3319 ppm | Murchison meteorite | ||
| Fullerenes >100 ppm | Murchison meteorite | ||
| Carboxylic acids >300 ppm | Murchison meteorite | ||
| Hydrocarboxylic acids 15 ppm | Murchison meteorite | ||
| Alcohols 11 ppm | Murchison meteorite | ||
| Nucleobases 1.3 ppm | Purines | Adenine | Murchison meteorite |
| Guanine | Murchison meteorite | ||
| Xanthine | Murchison meteorite | ||
| Pyrimidines | Uracil | Murchison meteorite | |
| Alkyl phosphonic acids 2 ppm | Ethylphosphonic acids | Murchison meteorite (Cooper &al., 1992) | |
| Methylphosphonic acids | Murchison meteorite (Cooper &al., 1992) | ||
| Alkyl sulfonic acids 68 ppm | Murchison meteorite (Cooper &al., 1992) | ||
| Silicates | CM group & CI group carbonacous chondrites. | ||
| Oxides | Dihydrogen monoxide 3-22% | CM group & CI group carbonacous chondrites. | |
| Sulfides | CM group & CI group carbonacous chondrites. | ||
| Phosphates | Apatite | (Schwartz, 2006) | |
| Inorganic phosphate | Detected in the Murchison meteorite at about 25 micromoles per gram (Cooper &al., 1992) | ||
| Inorganic orthophosphate | Murchison meteorite (Cooper &al., 1992) | ||
| Schreibersite [(Fe, Ni)3P] | (Schwartz, 2006) | ||
| Whitlockite [Ca9(Mg, Fe)(PO4)6PO3OH] | (Schwartz, 2006) | ||
| Chlorapatite [Ca5(PO4)3Cl] | (Schwartz, 2006) | ||
| Aluminous spinel | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Aluminum | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Anorthite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Calcic pyroxene | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Calcium | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Fosterite-rich olivine | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Hibonite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Isovaline | Murchison meteorite | ||
| Melilite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. | ||
| Perovskite | Calcium-Aluminum-rich or Ca-Al-rich inclusions (CAIs) in carbonaceous chondrites such as the Murchison meteorite. |
Part II.a-2.
Ice Mantle Composition
| Compound | Source |
|---|---|
| Cytosine | Experiments with astrophysical ice analogues. |
| Dihydrogen monoxide | |
| Purine | |
| Pyrimidine | |
| Ribose | Experiments with astrophysical ice analogues. |
| Thymine | Experiments with astrophysical ice analogues. |
Part II.b.
Atmosphere
Part III
Methods
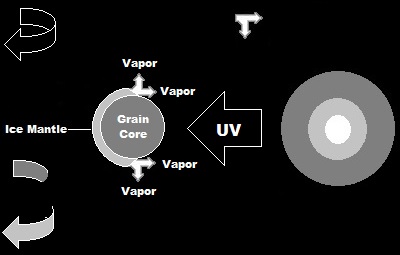
Ideally, the G3PAs would be of variable composition and each G3PA should not only be spinning on its own axis, but also moving around the source of the UV radiation in an orrery-like fashion so that each G3PA has to move through the sublimated material of the preceeding G3PA. This would simulate ice-mantled grains of variable composition in the protoplanetary Solar disk passing their sublimated material to grains following in their wake while acquiring such material from the grains proceeding them.